Many options include one component, referred to because the solvent, through which different components, referred to as solutes, are dissolved. An aqueous answer is one for which the solvent is water. The focus of an answer is a measure of the relative quantity of solute in a given quantity of solution. Concentrations could also be measured employing varied units, with one very helpful unit being molarity, outlined because the variety of moles of solute per liter of solution. The solute focus of an answer could also be decreased by including solvent, a course of often referred to as dilution. The dilution equation is an easy relation between concentrations and volumes of an answer earlier than and after dilution.
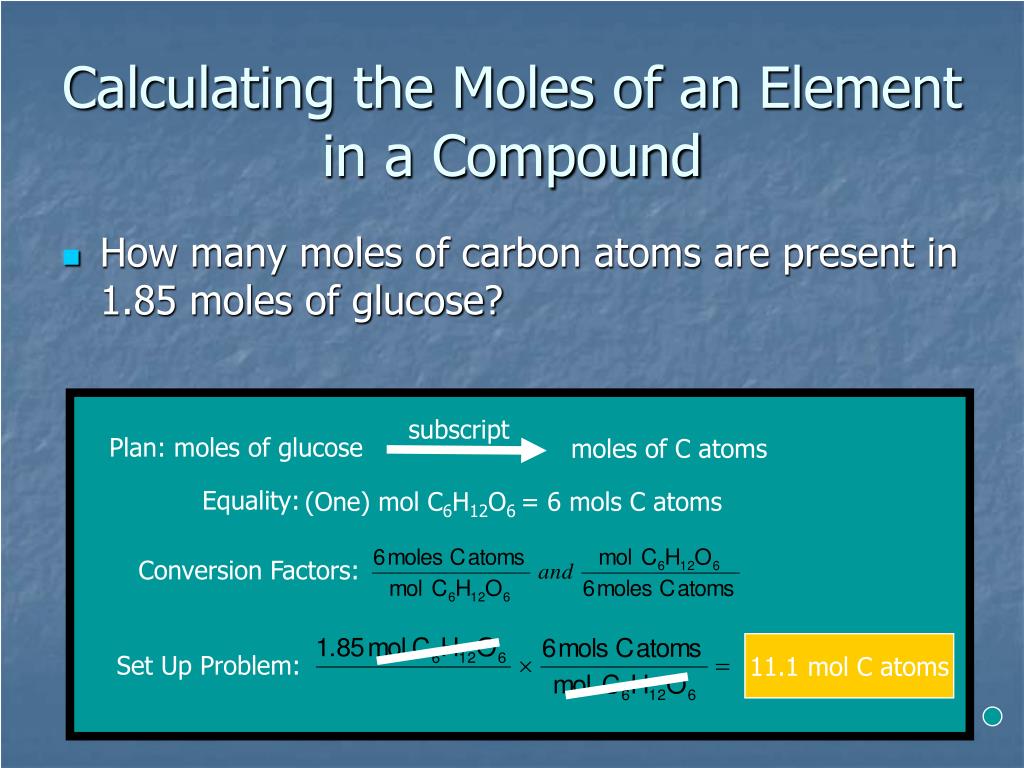
The relative formulation mass of a compound is calculated by including jointly the relative atomic mass values for all of the atoms in its formula. Moles are models used to measure substance amount. Molecular formulation are decided making use of atoms and ions. Learn about totally different molecular formulation and comprehend that ions can additionally decide formulas. Understand coefficients and ionic compounds, and use ionic compounds to elucidate molecular formulas. Find the variety of molecules in 4.48 cm3 of carbon dioxide fuel at commonplace temperature and pressure.
When 26.16 g of iron is heated within the presence of extra chlorine gas, they are going to react to type 76.08 g of a compound referred to as ntaining these 2 elements. If the molar mass of this compound is 644 g/mil discover the molecular formula. A mole is the usual unit quantity of a substance, an extremely significant number, often identified as Avogardo's number, what s it?
1 mole of a substance has the identical mass as its relative molecular mass. The molar mass of a compound refers back to the mass of the compound that's made up of 1 mole of the compound. It could very well be calculated making use of the molar plenty of the weather making up the compound. With a unit of g/mol, the molar mass of a compound helps decide the moles of compound current in a given mass. Molarity is the variety of moles of solute per liter of solution. A solute, which may be solid, liquid or gas, is a substance that's dissolved in a solvent.
The solvent is a different substance that's capable to dissolving it inside its intermolecular spaces. Together, the dissolved solute and the solvent make a solution. Is the method whereby the attention of an answer is lessened by the addition of solvent. For example, we'd say that a glass of iced tea turns into more and more diluted because the ice melts.
M OLARITY Is the variety of moles of solute dissolved per Liter of solution. Every aspect has a unique molar mass, often situated underneath the image on a periodic table. For example, one mole of carbon has a mass of 12.01 g/mol. The molar mass of hydrogen is 1.01 g/mol, and oxygen is 16.00 g/mol. While the counted quantity of a mole of any substance is 6.022 x 1023, the molar mass of that substance can be different. For example, sodium chloride, NaCl, may have a unique mass than desk sugar, sucrose, C12H22O11.
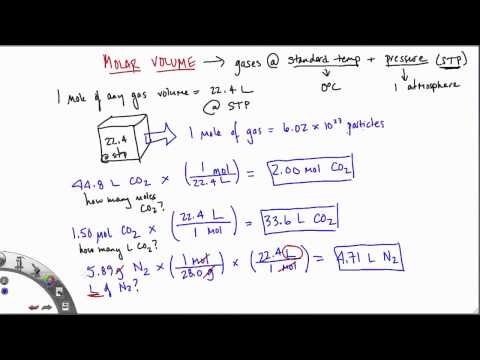
At the identical temperature and strain equal volumes of all gasses comprise the identical variety of molecules. Learn how one can calculate the variety of atoms in a pattern from the connection between moles and Avogadro's number. A pattern of argon, Ar, includes 5.97 moles of... Calculate the mass, in grams, of 6.15 moles of... Suppose the formulation mass of the salt was 200, calculate the molarity of the saturated solution.
A mole calculation in an answer requires making use of the molarity formula. The quantity of the answer and the answer focus is needed. Calculate the variety of moles of hydrogen current in a 500 cm3 pattern of hydrogen fuel at a strain of 760 mm Hg and 27°C. Learn about atomic mass units, abbreviated as AMUs. Learn the atomic mass unit definition, AMU measurements, and the way to transform AMUs to kilograms . There are 4 detailed steps that have to be taken when calculating the components mass of a whole molecule, which consists of a quantity of atoms.
Discover extra about formulation mass, gain knowledge of the four steps for calculating formulation mass, and uncover the carry out of mass spectroscopy. When performing calculations stepwise, as in Example 4, it really is very relevant chorus from rounding any intermediate calculation results, which may end outcome in rounding errors within the ultimate result. If we had not retained this guard digit, the ultimate calculation for the mass of NaCl would have been 77.1 g, a distinction of 0.3 g. STANDARD GRADE CHEMISTRY CALCULATIONS Calculations involving the mole.
1 mole of a stable substance is the formulation mass of the substance in grams. A pattern of 2.0 moles of dihydrogen fuel is positioned in a container with a quantity of 10.4 L. What is the strain of of the fuel in torr if the fuel is at 25 levels Celsius.
Molecular Mass by Freezing level Depression The following errors occurred when the above experiment was carried out. How would every impact the calculated molecular mass of the solute ? Learn the ideas of molar quantity and normal molar volume. See how one can calculate molar quantity and use the right molar quantity units. The molar mass of ammonium chloride, \rm NH_4Cl , is 53.49 g/mol. By subtracting the unique weight of the dish from the ultimate weight you get the mass of salt dissolved within the quantity or mass of saturated salt answer you began with.
The diagrams characterize two substances dissolved within the solvent, the right-hand diagram represents a extra concentrated answer e.g. a mixture of two salts in water. We must know the variety of moles of sulfuric acid dissolved within the answer and the quantity of the solution. Use the PhET simulation for Concentrationto discover the relations between solute amount, answer volume, and focus and to verify the dilution equation. Moles in Solution A answer consists of a solvent with a solute dissolved in it The focus of a options tells us how a lot solute is current in.
Moles and options By the top of part you need to have the opportunity to… Calculate the quantity of substance in mol, applying answer quantity and focus Describe. 1.1.7 Moles and Solutions Calculate the quantity of substance, in mol applying answer quantity and focus Describe a options focus applying the. Find the variety of moles of the substance you realize some of the most about Multiply by the stoichiometric ratio to get the number.
To have the ability to calculate the variety of moles of a solute in a solution, given the focus of the answer and the quantity of the solution. Calculate the molecular mass of sulphur if 35.5 g of sulphure dissolves in 1 00 g of CS2 to supply an answer that has a boiling level of 49.48 c. Calculate the molecular mass of sulphur if 35.5 g of sulphure dissolves in one hundred g of CS2 to supply an answer that has a boiling level of 49.48 c. One mole of any aspect or chemical compound is usually the identical number. One mole of hydrogen would imply there are 6.022 × 1023 atoms of hydrogen.
Report "Calculate the variety moles of hydrogen fuel current in 0.5 dm3 sample..." A formulation represents one molecule of a compound, or the only ratio of ions present. A wit symbols, a formulation represents a single particle or one mole of particles. Acids are substances that contribute molecules, whereas bases are substances that may settle for them.
Learn easy methods to outline acids and bases, discover the pH scale, and discover easy methods to outline pH values. Learn the definition, formula, and the way to calculate typical atomic mass. Understand the definition of a binary molecular compound. Learn the principles for naming binary molecular compounds and discover easy methods to write down their chemical formulas.
When a chemical response is balanced, each part of the equation have the identical wide variety and sort of atoms. In this lesson, discover the constituents of a chemical equation and discover ways to put in writing balanced chemical reactions. Solubility would be measured and expressed in with diverse focus models e.g. g/100cm3, g/dm3 and molarity (mol/dm3).
Use the triangle on the ideal that will enable you rearrange the equation for the essential definition of molarity. The focus of an aqueous answer given the quantity of substance and quantity of water, for this you employ the equation .... A 1.0 molar answer of magnesium chloride MgCl2, includes 1.0 mol/dm3 of magnesium ions (Mg2+), BUT 2.0 mol/dm3 when it comes to the chloride ion (Cl-) concentration.
How To Calculate The Number Of Moles Of Gas Present First convert mass in grams to moles, after which substitute the right phrases into the definition. Dilution is additionally a standard technique of getting ready options of a desired concentration. By including solvent to a measured portion of a extra concentrated inventory solution, we will obtain a specific concentration. For example, business pesticides are usually bought as options wherein the lively components are much extra concentrated than is acceptable for his or her application.
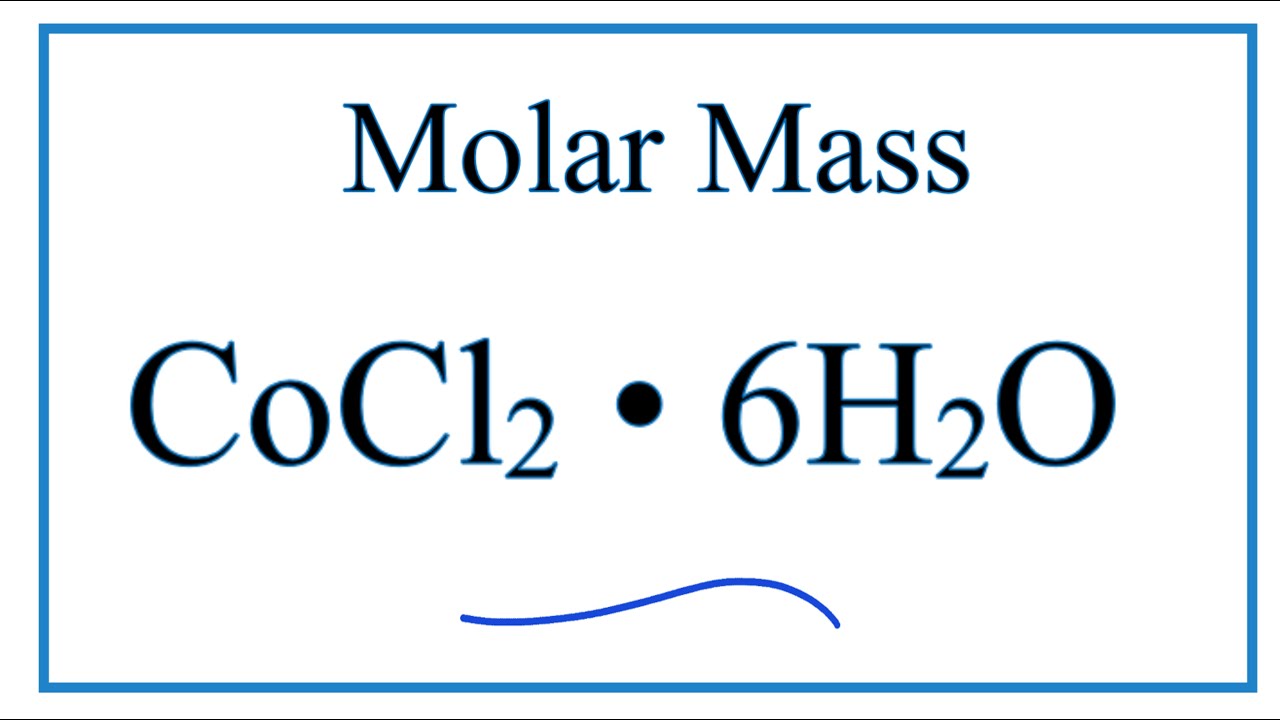
Before they are often used on crops, the pesticides have to be diluted. This is usually a quite typical apply for the preparation of a variety typical laboratory reagents . Calculate the molarity of 6.52 g of CoCl2 (128.9 g/mol) dissolved in an aqueous answer with a complete quantity of 75.0 mL. We have until now outlined options as homogeneous mixtures, which means that the composition of the combination is uniform all by using its complete volume. Solutions take place regularly in nature and have additionally been carried out in lots of types of artifical technology.
We will discover a extra thorough healing of answer properties within the chapter on options and colloids, however right here we'll introduce among the essential properties of solutions. A pattern of xenon fuel at a strain of 704 mm Hg and a temperature of forty five °C, occupies a quantity of 12.6 liters. If the fuel is cooled at fixed strain to a temperature of 21 °C, the quantity of the fuel pattern will be___ L.
If a mole of bagels have been purchased, they might just about fill the inside area of the Earth. Although a mole of some factor might possibly be counted, it really is typically reserved for extremely small items, like atoms and molecules. Seltzer water is made by dissolving CO2 in water. Seltzer might possibly be made at house employing small containers of pressurized CO2.
With this instance we will clearly see the connection between the variety of moles of a gas, and the quantity of a gas. Now we now should transform the 3.0 mol of HCl into grams of HCl. This will be achieved by multiplying 3.0 mol by the molecular weight of HCl, which is 36.46 g/mol. Calculate the strain of a fuel provided that 0.2 moles of the fuel occupy 10dm3 at 20 levels C.
This is a kind of covalent bond the place equally of the shared pair of electrons come from the identical atom. Ammonia molecule NH3 can bond with a H+ ion to type Ammonium ion NH4+. Ionic compounds don't use the prefixes di, tri, and so on in the event you see these the compound should be _____. If the compound ends in ate, ite and so on there should be a polyatomic ion, for this reason the compounds is ____. I.e sodium and chlorine can be sodium chloride. What is a molecule sharing electrons however with no cost resulting from no switch of electrons.
Metals mostly obtain electrons however as they can't each obtain electrons they share them. Understand the definition of atomic mass, atomic number, and atomic weight. Discover methods to define atomic mass, and obtain knowledge of what an atomic variety represents. See atomic mass examples, obtain knowledge of the atomic mass definition, methods to define the atomic mass of an atom, and the significance of atomic mass. OR, a phial of concentrated acid or alkali, which you dilute right into a specified quantity to provide a selected molarity.
To put together an answer of identified molarity, you'll want to work backwards from the quantity required and the molarity to see how a lot stable you need. However, many substances like salts are very soluble in water and an easy evaporation technique will do which is described under e.g. for a thermally secure salt like sodium chloride. The solubility of a substance is the utmost quantity of it that can dissolve in a given quantity of solvent e.g. water. You have to know all about moles to proceed additional on this web page and get into 'molarity' ... Using attention models of mol dm-3 (or mol/dm3), the attention identified as molarity, from time to time denoted in shorthand as M (old funds again, take care!) and the phrase molar is used too.
A low power answer of acid shows it's a weak acid and solely ionises a number of percent to provide hydrogen ions. You can then do a molarity calculation to envision the molarity of the unknown concentration. Molarity Molarity or molar attention is a measure of the attention of a solute in a solution. If you need to know the variety of moles of a solute the place there are 500 cm3 of a 2.0 mol.dm-3 solution...
Calculate the method wright of a 2.50 g pattern of fuel whose quantity is 1.40l at STP. So, the quantity of a really perfect fuel is 22.41 L/mol at STP. Initially the quantity of the piston is 3.0 L, and the strain of the fuel is 5.0 atm.
The piston is used to compress the fuel to a quantity of 1.5 L; decide the strain of the N2O. When a metallic atoms bonds with a non-metal atom the metallic atom transferred electrons to the non-metal to type a ____ metallic ion and a ____ non-metal ion. Usually shaped when atoms obtain or lose electrons.
Gibbs Free Energy and cell potential vigor measure the spontaneity of electrochemical reactions as vigor is changed from chemical to electrical. Learn about galvanic cells, methods to measure cell potential energy, and free vigor vs. cell potential energy. To make certain each drop of the salt answer results within the flask, a wash bottle of pure water is used to rinse out the beaker a number of times, AND rinse the stirring rod and the funnel too. 2.295g of pure NaCl salt is required to made up 250.0 cm3 of answer with a exact attention of 0.20 mol/dm3.